Recently, the Professor Zhongbin Zhuang from the College of Chemical Engineering and collaborators have made progresses on hydroxide exchange membrane fuel cells (HEMFC). Two research articles titled "Atomically dispersed Iridium on Mo2C as an efficient and stable alkaline hydrogen oxidation reaction catalyst" and "Tuning the apparent hydrogen binding energy to achieve high-performance Ni-based hydrogen oxidation reaction catalyst" are published in Nature Communications. These two studies addressed the issues of low activity and high cost of anodic catalysts in HEMFC by designing highly active and stable hydrogen oxidation catalytic materials with low usage of precious metals and non-precious metals.
HEMFCs have garnered extensive attention due to their cost benefits, such as the use of affordable non-precious metal cathode catalysts and nickel-based bipolar plates. However, high-performance HEMFCs still heavily rely on precious metal catalysts at anode side, because the kinetics of the hydrogen oxidation reaction (HOR) catalyzed by precious metal catalysts are significantly slowed down under alkaline conditions. Therefore, developing highly efficient and stable catalyst to lower precious metal requirement on anode is important to reduce the cost of HEMFCs.
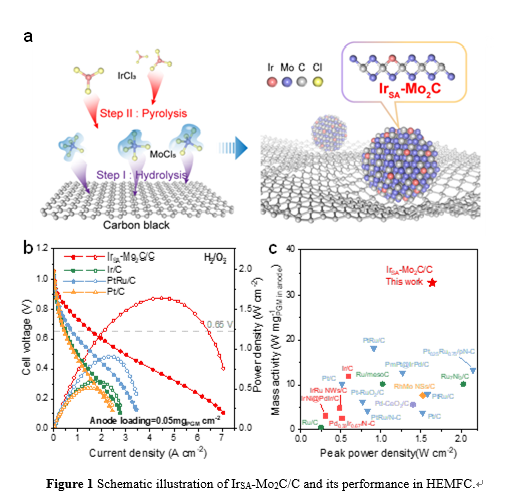
Single-atom catalysts have high noble metal utilization efficiency. However, carbon-based single-atom catalysts typically exhibit low HOR catalytic activity and cannot meet the anode requirements of HEMFCs. The research group utilizes the Mo2C nanoparticle as substrate to prepare atomically dispersed Ir (IrSA-Mo2C/C) due to the Pt-like band structure of Mo2C and its strong interaction with noble metals, achieving highly active and stable alkaline HOR catalysis. The specific exchange current density of IrSA-Mo2C/C is 4.1 mA cm−2ECSA, which is 10 times that of Ir/C. Negligible decay is observed after 30,000-cycle accelerated stability test. Theoretical calculations suggest the high HOR activity is attributed to the unique Mo2C substrate, which makes the Ir sites with optimized H binding and also provides enhanced OH binding sites. By using a low loading (0.05 mgIr cm−2) of IrSA-Mo2C/C as anode, the fabricated HEMFC can deliver a high peak power density of 1.64 W cm−2. This work illustrates that atomically dispersed precious metal on carbides may be a promising strategy for high performance HEMFCs.
Ni-based catalysts exhibit potential catalytic capabilities for the HOR in alkaline media. However, their activity levels fail to meet the demands for constructing high-performance HEMFC. The research group has developed a Cr2O3-modified NiCu alloy (NiCuCr/C) catalyst through a dual strategy involving the regulation of electrode components and the optimization of the interface water structure in electric double layer, achieving high activity and stability for HOR catalysis. The HEMFC using NiCuCr/C as anode with a high peak power density of 577 mW cm−2 (18 times as the HEMFC use Ni/C anode) and more than 150 h of stability (degradation rate slow by 7 times than the HEMFC use Ni/C anode). The spectroscopies demonstrate the alloy effect from Cu to weaken the hydrogen binding and the surface Cr2O3 species to enhance the interfacial water binding. Both effects bring an optimized apparent hydrogen binding energy, and thus led to the high HOR performance of NiCuCr. These results suggest the apparent hydrogen binding energy determined the HOR performance and its tuning is beneficial towards high electrocatalytic performance.
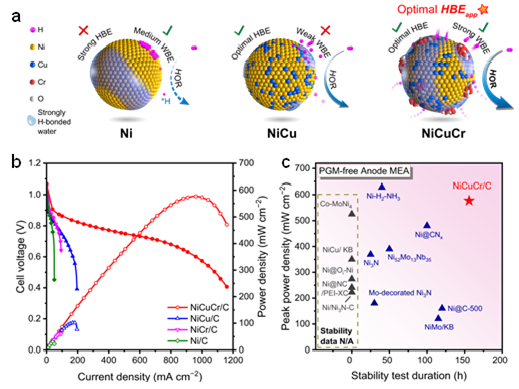
Figure 2 Schematic illustration of NiCuCr/C and its performance in HEMFC
The link for the IrSA-Mo2C/C paper is https://www.nature.com/articles/s41467-024-48672-9. Jinjie Fang, Haiyong Wang, and Qian Dang from Beijing University of Chemical Technology are the co-first authors. Professors Zhongbin Zhuang and Hui Li from Beijing University of Chemical Technology, and Professor Yushan Yan from the University of Delaware are the co-corresponding authors.
The link for the NiCuCr/C paper is https://www.nature.com/articles/s41467-024-45370-4.Xingdong Wang, Xuerui Liu, and Jinjie Fang are the co-first authors. Professor Zhongbin Zhuang is the corresponding author.
