Recently, a research team from State Key Laboratory of Organic-Inorganic Composites in Beijing University of Chemical Technology (BUCT) published a research paper in Nature Sustainabilityentitled “water-in-polymer electrolyte with a wide electrochemical window and recyclability”.The research was focused on the coordiantion structure of H2O, and analyzed the factors that influenced the electrochemcial window (EW) of aqueous electrolytes, from the perspective of both thermodynamics and dynamics. By using water-in-polymer structure, together with in situ generating passivation layers on anode, the aqueous solid-state electrolytes with low salt concentration were endowed with wide EWs. Furthermore, a strategy for recycling lithium salt was also propsoed. The electrolyte design represents a substantial step forward towards more sustainable aqueous batteries.
The chasing of larger single cell and higher energy density for Li-ion batteries (LIBs) raises the concern of safety issues. A promising solution is to replace the organic electrolytes with aqueous electrolytes, which also contributes to reducing the manufacturing cost and protecting environment. However, their practical applications have been largely limited by the intrinsic narrow EW, that is, the high electrochemical potential of hydrogen evolution reaction (HER) at the anode side. Constructing water-in-salt (WIS) concentrated aqueous electrolytes could extend the EW to over 3.2 V. However, the excessive use of expensive Li salt not only increases the cost but also leads to environmental issues. As a compromise, introducing organic solvents into WIS electrolytes to form locally oversaturated Li-salt systems can address the problems to some extent. Nevertheless, the partial replacement of water with flammable organic solvents is inevitably at the cost of safety.
Here, a water-in-polymer solid electrolyte (WIPSE) was constructed, which maximized the amount of water but worked across a voltage range as wide as that for highly concentrated electrolytes. At the heart of this formulation was the introduction of a polyacrylamide network that served to immobilize and thus tame the otherwise reactive H2O molecules. The coordination structure of H2O, which determined the EWs of aqueous electrolytes, was probed from both thermodynamical and dynamical perspectives. Thermodynamically, hydrogen bonds elongated and weakened O–H bonds. In comparison, the Li+–O interaction could enhance the strength of O–H bonds and was more dominant than hydrogen bonds. Dynamically, both hydrogen bonds and Li+–O interaction helped confine H2O and suppress HER. As a result, owing to the dynamical confinement of H2O by hydrogen bonding with polymer networks, the thermodynamical strengthening of O–H bonds by Li+–O interaction, and the generation of a passivating layer on anode, a low-concentration and high-water-content aqueous electrolyte could be endowed with limited interfacial side reactions and enabled stable cycling of high-voltage solid-state cells.
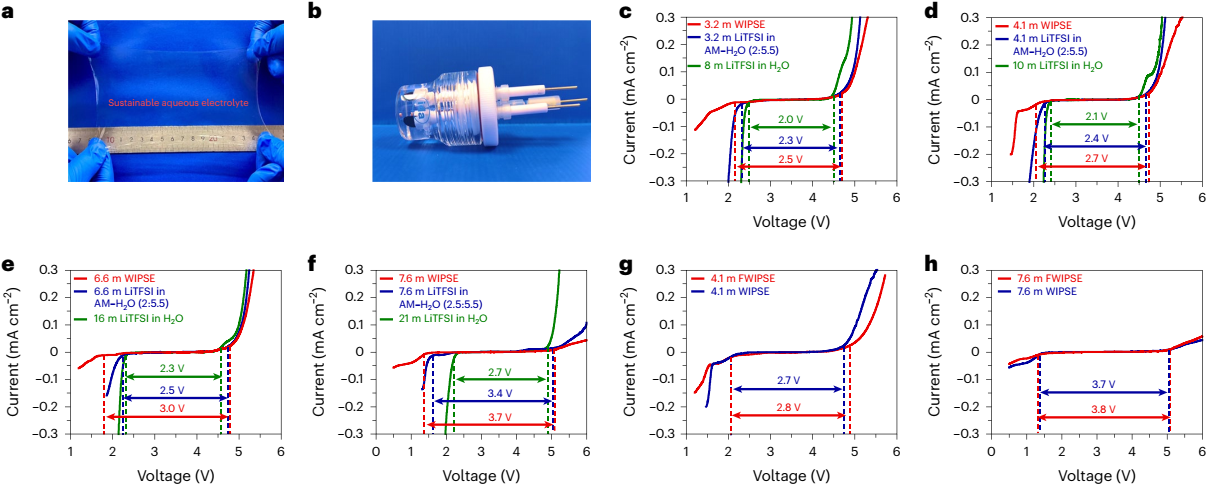
Fig 1. EW tests of WIPSEs and FWIPSEs.
By constructing a robust polymer network to confine H2O through multiple hydrogen bonds, the WIPSEs with low concentrations of 4.1 m and 7.6 m exhibited extended EWs of 2.7 V and 3.7 V, comparable to that of the saturated 21 m LiTFSI in H2O and locally oversaturated 40 m LiTFSI in H2O, respectively. The solid-state Mo6S8//LiMn2O4 cell with 4.1 m WIPSE (18 wt% H2O) delivered substantially improved cycling stability than that with unsolidified 4.1 m WIPSE-precursor electrolyte. With the co-polymerization of 5 mol% TFMA, hydrophobic F-containing SEI was intensified on anode, which further suppressed the interfacial reactions on anode and enabled a long-term stable cycling of Mo6S8//LiMn2O4 in a lower P/N of 1.3. By further increasing the Li-salt concentration and immobilizing H2O, 7.6 m F-containing WIPSE (FWIPSE) with 11 wt% H2O enabled stable cycling of Li4Ti5O12//LiMn2O4 cells, even in a higher LiMn2O4 loading of 16 mg cm-2 with less electrolyte (E/C = 7 g Ah-1). Furthermore, owing to the decreased LiTFSI consumption and limited side reactions of solid-state electrolytes, 77–80% of the highly expensive LiTFSI can be recycled, and the FWIPSEs could also be regenerated. The study is expected to inspire more work on sustainable LIBs with reduced cost and improved environmental friendliness.
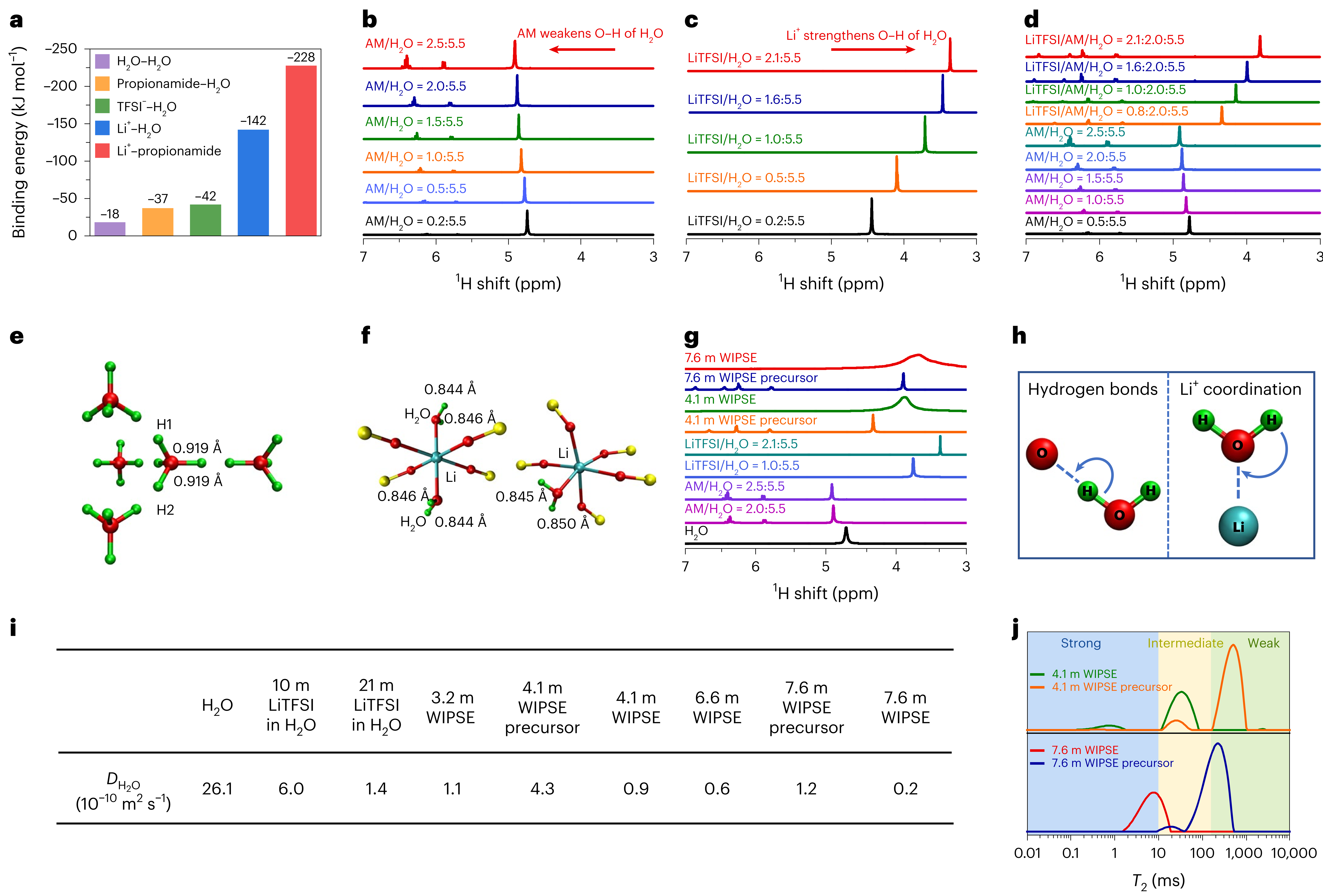
Fig 2. Hydrogen bonds and Li+–O interaction in WIPSEs
The first author is associate professor Shu-Meng Hao from BUCT. The co-first authors are Jianxun Zhu, Shuang He, and Le Ma, PhD students from BUCT. The corresponding authors are Prof. Liqun Zhang (member of Chinese Academy of Engineering), and Prof. Weidong Zhou from BUCT.
Corresponding link: https://www.nature.com/articles/s41893-024-01327-5
