Prof. Yue Feng’s group published an Article
in Nature Communications
On April 14, 2020, Prof. Yue Feng's research group published a research article in Nature Communications, entitled Structural insights into the mechanism and inhibition of transglutaminase-induced ubiquitination by the Legionella effector MavC, which reported the molecular mechanism of the ubiquitination reaction catalyzed by the transglutaminase MavC of Legionella pneumophila and its inhibition.
Protein ubiquitination is one of the most prevalent post-translational modifications, controlling almost every process in eukaryotic cells. Legionella pneumophila (L. pneumophila) is the causative agent of a severe, potentially fatal pneumonia known as Legionnaires’ disease in humans. Based on the important functions of ubiquitination, L. pneumophila can manipulate the ubiquitination process of its host through multiple mechanisms during host-microbe interactions. In 2018, Yue Feng’s group had studied the molecular mechanism of an unconventional ubiquitination mediated the Legionella effector SdeA. This finding was published in Nature.
MavC is another Legionella effector which harbors transglutaminase activity (Figure 1a). MavC could mediate a unique ubiquitination through transglutamination, linking ubiquitin (Ub) to UBE2N through UbGln40, which can be inhibited by another Legionella effector Lpg2149. In the present study, Yue Feng’s group solved the structures of MavC/UBE2N/Ub ternary complex (Figure 1b), MavC/UBE2N-Ub (product) binary complex (Figure 1c), and MavC/Lpg2149 binary complex (Figure 1d). They found that, during the ubiquitination, the loop containing the modification site K92 of UBE2N undergoes marked conformational change and Lpg2149 inhibits this ubiquitination through competing with Ub to bind MavC. Interestingly, during the co-crystallization process of MavC and UBE2N-Ub, they found that MavC itself also exhibits weak deubiquitinase activity towards this non-canonical ubiquitination. This led them to test whether the homolog Together, their study not only provides unprecedented insights into the mechanism and inhibition of this transglutaminase-induced ubiquitination by MavC, but also sheds light on the future studies into UBE2N inhibition by this modification and deubiquitinases of this unique ubiquitination.
Yajuan Mu, Yue Wang, Yanfei Huang and Dong Li are the co-first authors of this paper, and Prof. Yue Feng is the corresponding author of this paper. This work was accomplished by Beijing Soft Matter Science and Engineering Top Innovation Center and Beijing University of Chemical Technology. Prof. Zhao-Qing Luo and Dr. Jiaqi Fu of Purdue University participated in the work and provided much help for the project. Beamlines BL17U1 and BL19U1 of the National protein science research (shanghai) facility and their staff provided timely and effective support for data collection. This research work was supported by the National Natural Science Foundation, the National Key Research and Development Program and Beijing University of Chemical Technology.
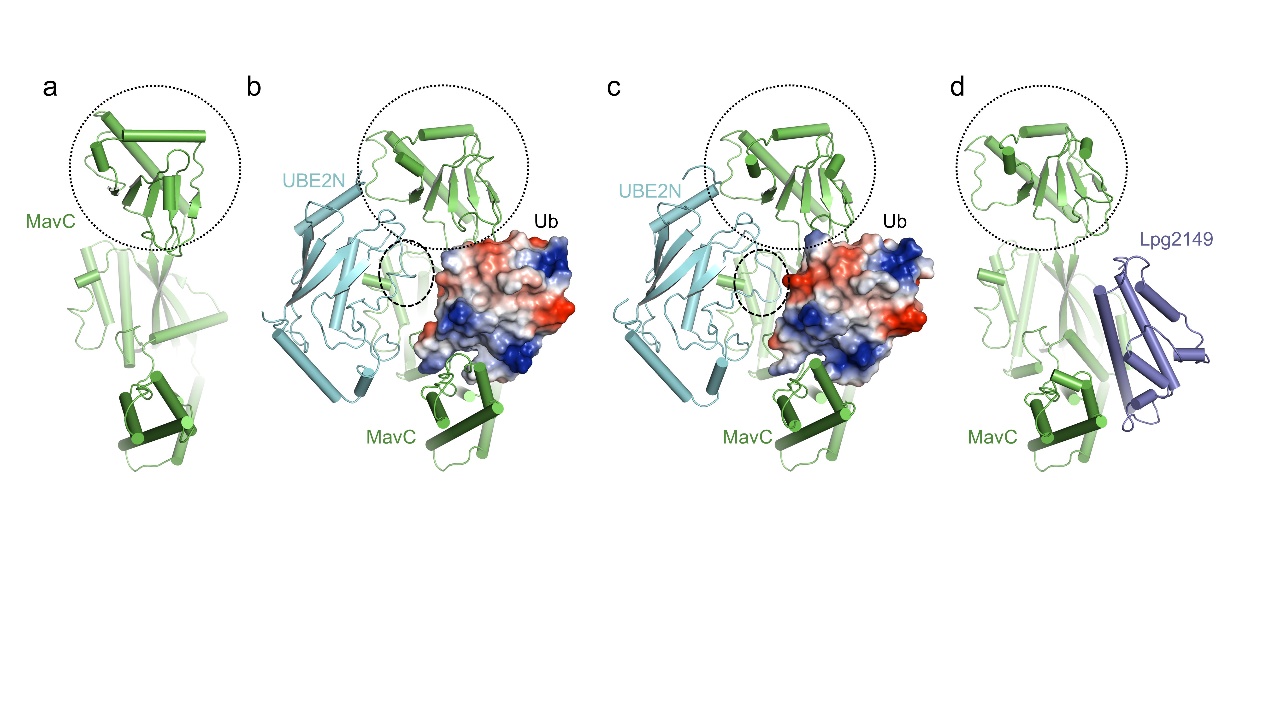
Figure 1 Structures involved in the study
The structures of MavC (a), MavC/UBE2N/Ub (b), MavC/UBE2N-Ub (c) and MavC/Lpg2149 (d). Ub is shown as an electrostatic model in all structures. The insertion domain of MavC and the K92 loop region of UBE2N are marked with circles.
